2025
Effect of ultra-processed food consumption on male reproductive and metabolic health
Preston JM, Iversen J, Hufnagel A, Hjort L, Taylor J, Sanchez C, George V, Hansen AN, Ängquist L, Hermann S, Craig JM, Torekov S, Lindh C, Hougaard KS, Nóbrega MA, Simpson SJ, Barrès R. Cell Metab. 2025 Aug 22:S1550-4131(25)00360-2. doi: 10.1016/j.cmet.2025.08.004
https://www.cell.com/cell-metabolism/fulltext/S1550-4131(25)00360-2
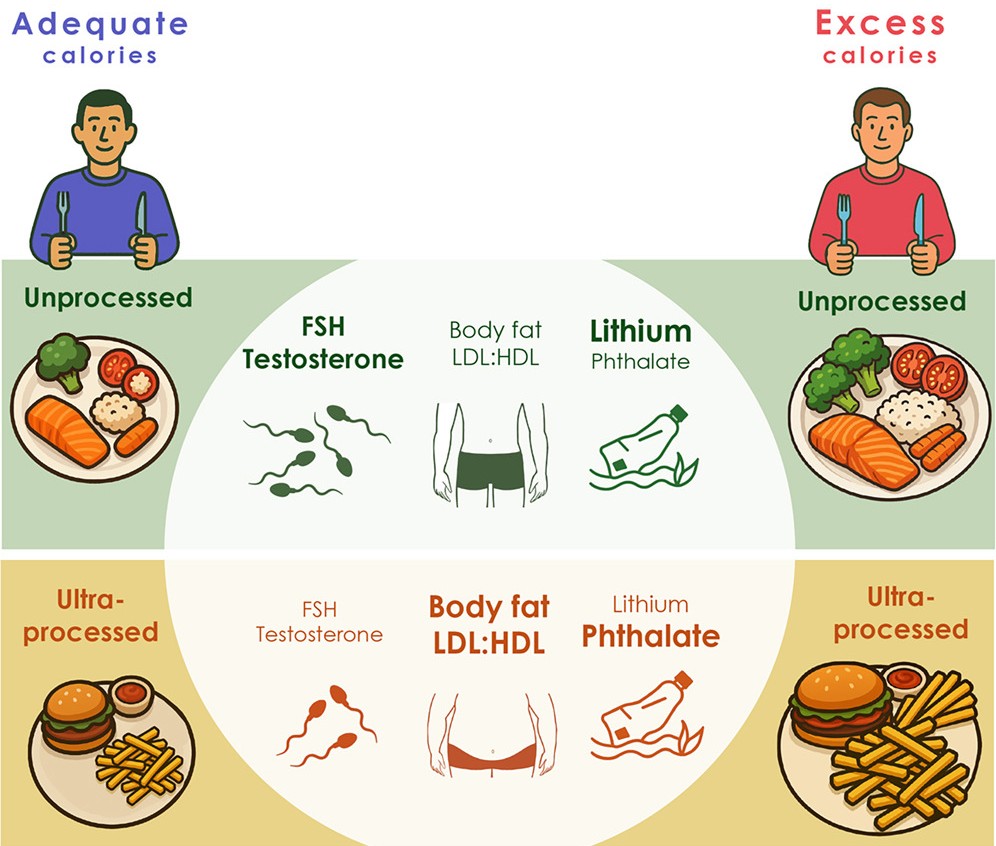
Highlights
- Compared with an unprocessed diet, UPF impaired cardiometabolic and reproductive health
- The deleterious effects of a UPF diet were independent of total caloric intake
- A UPF diet altered the balance of several hormones, including GDF-15 and FSH
- A UPF diet was associated with higher serum concentration of the phthalate cxMINP
Consumption of ultra-processed food is associated with increased caloric intake and impaired health. Here, we conducted a nutrition trial (NCT05368194) with controlled, 2 × 2 crossover design and tested whether ultra-processed food impairs reproductive and metabolic fitness, with further aggravation by excess caloric intake. Comparing the response from an unprocessed to ultra-processed diet identified increased body weight and low-density lipoprotein (LDL):high-density lipoprotein (HDL) ratio, independent of caloric load. Several hormones involved in energy metabolism and spermatogenesis were affected, including decreased levels of growth/differentiation factor 15 and follicle-stimulating hormone. Sperm quality trended toward impairment, with a decrease in total motility. Differential accumulation of pollutants between the discordant diets were detected, such as decreased plasma lithium and a trend for increased levels of the phthalate mono(4-methyl-7-carboxyheptyl)phthalate (cxMINP) in serum, following the ultra-processed diet. Alteration in caloric load alone had distinct effects on the measured outcomes. This study provides evidence that consumption of ultra-processed food is detrimental for cardiometabolic and reproductive outcomes, regardless of excessive caloric intake.
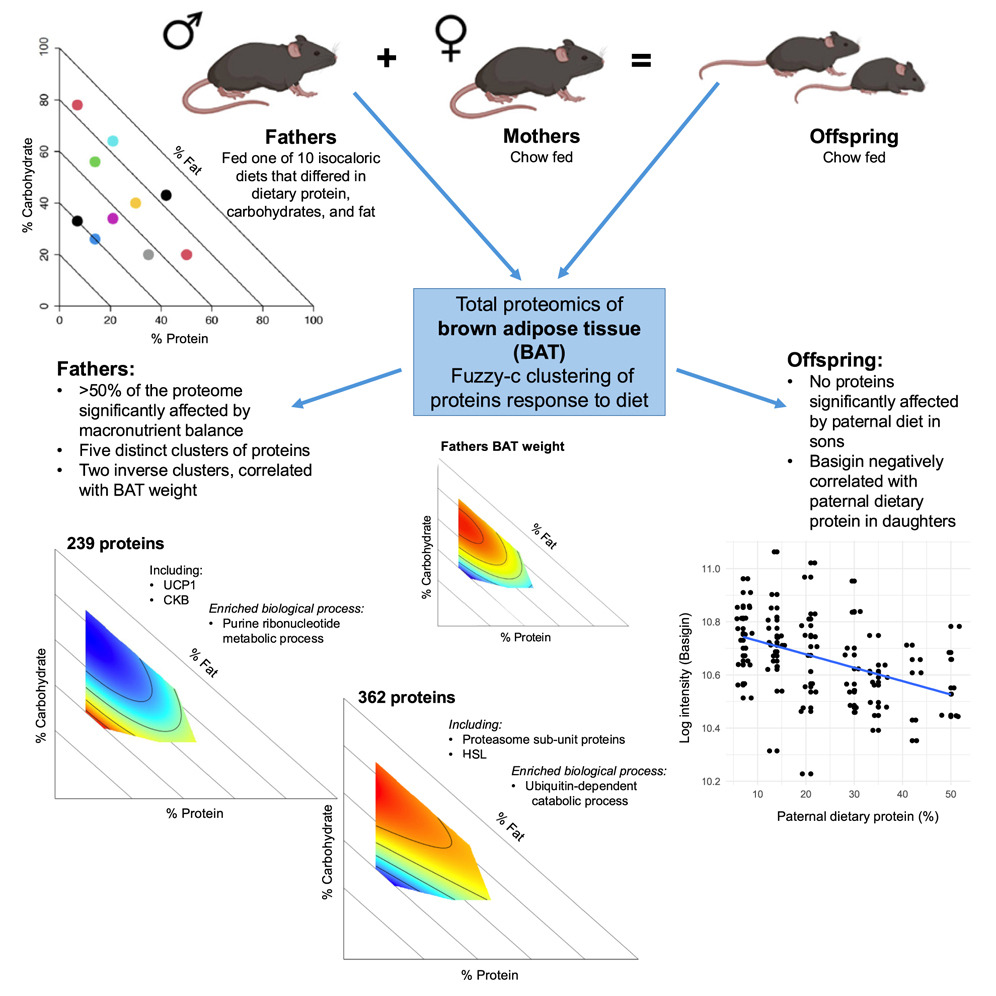
Dietary macronutrients modulate the proteome of brown adipose tissue in males and their female offspring.
Macartney EL, Senior AM, Crean AJ, Small L, Pulpitel TJ, Ruffino L, Nobrega MA, Barrès R, Simpson SJ. Cell Reports. 2025 Jul 24;44(8):116050
Dietary macronutrient composition in males influences brown adipose tissue (BAT) size and BAT size of daughters in C57BL/6J mice. However, the effects of macronutrients and paternal effects on BAT function have yet to be characterized. We investigated the effects of macronutrient composition on the BAT proteome in male mice and offspring. In fathers, >50% of the proteome was affected by macronutrients. We identified two clusters with inverse patterns that correlated with BAT mass. Notably, uncoupling protein 1 (UCP1) was reduced on low-fat diets that promoted increased BAT mass, while there were increased levels of proteins involved in protein turnover. The same diets also led to a reduction in proteins involved in purine biosynthesis (purines are often UCP1 inhibitors). We also found that paternal protein intake negatively affected basigin expression in daughters, a protein that regulates Ucp1 transcription. Our results show that macronutrients in males remodel the protein expression of BAT directly and in their daughters.
https://www.sciencedirect.com/science/article/pii/S2211124725008216?via%3Dihub
Healthy lifestyle before and during pregnancy to prevent childhood obesity – study protocol for a parallel group randomised trial – The PRE-STORK trial.
Kornerup N, Danielsen JH, Sahl RE, Pico ML, Johansen MY, Knop FK, Bønnelykke K, Bergholt T, Kelstrup L, Foghsgaard S, Ghauri N, Grønlund E, Lund L, Vinter CA, Forman JL, Barrès R, Nielsen KK, Andersen A, Torekov SS, Grunnet LG and Vilsbøll T. BMJ Open. 2025 Jan 25;15(1):e087895.
https://bmjopen.bmj.com/content/15/1/e087895.long
Introduction: The global prevalence of people living with overweight has tripled since 1975 and more than 40% of Danish women enter pregnancy being overweight. With the increasing rates of obesity observed in children, adolescents and adults, there is an urgent need for preventive measures. Risk factors for childhood obesity include maternal overweight or obesity before conception and excessive weight gain during pregnancy. Interventions aimed at modifying maternal lifestyle during pregnancy have demonstrated minimal positive or no impact on the health of the children. The ‘healthy lifestyle before and during pregnancy to prevent childhood obesity – the PRE-STORK trial’ aims to provide insights into the effect of a lifestyle intervention initiated before conception and continued during pregnancy in women with overweight or obesity, on neonatal adiposity in their children.
Methods and analysis: In this randomised, two-arm, parallel-group, controlled trial, we will include 360 women with overweight or obesity (aged 18-40; body mass index 25-44 kg/m2) and their partners. The women will be randomised to receive either standard of care or a lifestyle intervention focused on preconception body weight reduction, regular physical exercise, healthy diet and support from a mentor before and during pregnancy. The primary outcome is the difference in neonatal adiposity measured in their children at birth. Children conceived during the trial will constitute a birth cohort, monitoring the effects on their health until the age of 18 years.
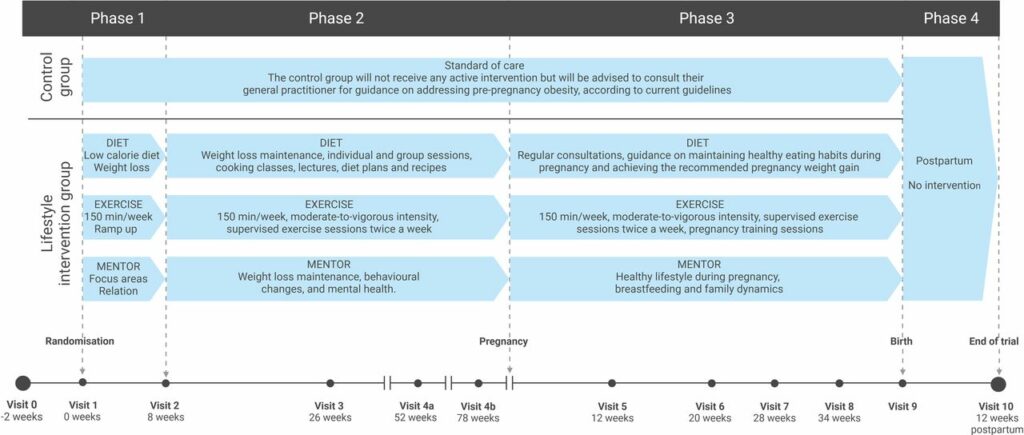
Ethics and dissemination: The trial has been approved by the Regional Committee on Health Research Ethics in the Capital Region of Denmark (identification number H-22011403) and will be conducted in agreement with the Declaration of Helsinki. All results, whether positive, negative and inconclusive, will be disseminated at national or international scientific meetings and in peer-reviewed scientific journals.
Trial registration number: ClinicalTrials.gov: NCT05578690 (October 2022).
A Guinea Pig Model of Pediatric Metabolic Dysfunction-Associated Steatohepatitis: Poor Vitamin C Status May Advance Disease
Pedersen K, Poojari A, Colberg SF, Mechernsee SM, Iversen JF, Barrès R, Lykkesfeldt J, Tveden-Nyborg P. Nutrients.2025 Jan 15;17(2):291.
https://www.mdpi.com/2072-6643/17/2/291
Background/Objectives: Children and teenagers display a distinct metabolic dysfunction-associated steatohepatitis (MASH) phenotype, yet studies of childhood MASH are scarce and validated animal models lacking, limiting the development of treatments. Poor vitamin C (VitC) status may affect MASH progression and often co-occurs with high-fat diets and related metabolic imbalances. As a regulator of DNA methylation, poor VitC status may further contribute to MASH by regulating gene expression This study investigated guinea pigs—a species that, like humans, depends on vitC in the diet—as a model of pediatric MASH, examining the effects of poor VitC status on MASH hallmarks and global DNA methylation levels. Methods: Sixty-two juvenile guinea pigs were exposed to a high-fat diet for 16 weeks. Results: Juvenile guinea pigs exhibited hepatic histopathology representative of pediatric MASH, confirmed by portal inflammation and fibrosis. Consistent with pediatric MASH, juvenile guinea pigs displayed increased lobular and portal inflammation (p < 0.05 and p < 0.0001, respectively) but less steatosis (p < 0.001) compared to adults. Compared to the controls, the guinea pigs deprived in VitC showed lower body weight (p < 0.01), higher expression of hepatic inflammatory genes (p < 0.05), and a lower global hydroxymethylcytosine to methylcytosine ratio in the high-fat groups (p < 0.05). Conclusions: Our study validates guinea pigs as a model of pediatric MASH and suggests that VitC contributes to an altered gene expression signature through the regulation of DNA hydroxymethylation. We postulate that nutritional co-deficiencies in MASH, such as low VitC, may accelerate disease progression and deserve further attention.
Exposure to childhood maltreatment is associated with specific epigenetic patterns in sperm.
Tuulari, JJ, Bourgery, M, Iversen, J, Koefoed, TG, Ahonen, A, Ahmedani A, Kataja EL, Karlsson L, Barrès R*, Karlsson L*, Kotaja, N*. Molecular Psychiatry. 2025 Jun;30(6):2635-2644.
https://www.nature.com/articles/s41380-024-02872-3#Fig1
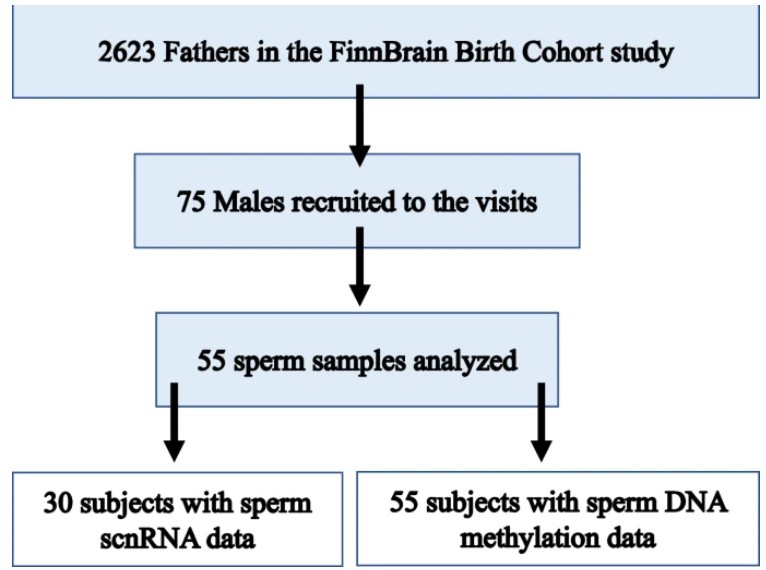
Childhood maltreatment exposure (CME) increases the risk of adverse long-term health consequences for the exposed individual. Animal studies suggest that CME may also influence the health and behaviour in the next generation offspring through CME-driven epigenetic changes in the germ line. Here we investigated the associated between early life stress on the epigenome of sperm in humans with history of CME. We measured paternal CME using the Trauma and Distress Scale (TADS) questionnaire and mapped sperm-borne sncRNAs expression by small RNA sequencing (small RNA-seq) and DNA methylation (DNAme) in spermatozoa by reduced-representation bisulfite sequencing (RRBS-seq) in males from the FinnBrain Birth Cohort Study. The study design was a (nested) case-control study, high-TADS (TADS ≥ 39, n = 25 for DNAme and n = 14 for small RNA-seq) and low-TADS (TADS ≤ 10, n = 30 for DNAme and n = 16 for small RNA-seq). We identified 3 genomic regions with differential methylation between low and high-TADS and 68 tRNA-derived small RNAs (tsRNAs) and miRNAs with different levels in males with high CME (False discovery rate, FDR corrected p < 0.05). Of potential interest, we identified differential expression of miRNA hsa-mir-34c-5p and differential methylation levels near the CRTC1 and GBX2 genes, which are documented to control brain development. Our results provide further evidence that early life stress influences the paternal germline epigenome and supports a possible effect in modulating the development of the central nervous system of the next generation.
2024
Extracellular vesicles – small messengers with a wide range of applications?
Hufnagel A, Barrès R. Dan Med J 2024 Jul; 71(8)
https://ugeskriftet.dk/dmj/extracellular-vesicles-small-messengers-wide-range-applications
Extracellular vesicles (EVs) are small membranous vesicles secreted by many different cell types that have emerged as potentially important mediators of organ crosstalk. EVs are of research interest in health and disease and as biomarkers and therapeutic agents in various fields, including metabolism, reproduction, cancer, and others. Despite promising data and a growing understanding of their role, challenges and limitations of EV research remain, leaving room for optimisation regarding methods for pure isolations of EVs and translation into clinical practice.
Epigenetics of the non-coding RNA nc886 across blood, adipose tissue and skeletal muscle in offspring exposed to diabetes in pregnancy.
Hjort L, Bredgaard SS, Manitta E, Marques I, Sørensen AE, Martino D, Grunnet LG, Kelstrup L, Houshmand-Oeregaard A, Dalsgaard Clausen T, Mathiesen ER, Olsen SF, Saffery R, Barrès R, Damm P, Vaag AV, Dalgaard LT. Clinical Epigenetics. 2024 May 7;16(1):61.
Background
Diabetes in pregnancy is associated with increased risk of long-term metabolic disease in the offspring, potentially mediated by in utero epigenetic variation. Previously, we identified multiple differentially methylated single CpG sites in offspring of women with gestational diabetes mellitus (GDM), but whether stretches of differentially methylated regions (DMRs) can also be identified in adolescent GDM offspring is unknown. Here, we investigate which DNA regions in adolescent offspring are differentially methylated in blood by exposure to diabetes in pregnancy. The secondary aim was to characterize the RNA expression of the identified DMR, which contained the nc886 non-coding RNA.
Methods
To identify DMRs, we employed the bump hunter method in samples from young (9–16 yr, n = 92) offspring of women with GDM (O-GDM) and control offspring (n = 94). Validation by pyrosequencing was performed in an adult offspring cohort (age 28–33 years) consisting of O-GDM (n = 82), offspring exposed to maternal type 1 diabetes (O-T1D, n = 67) and control offspring (O-BP, n = 57). RNA-expression was measured using RT-qPCR in subcutaneous adipose tissue and skeletal muscle.
Results
One significant DMR represented by 10 CpGs with a bimodal methylation pattern was identified, located in the nc886/VTRNA2-1 non-coding RNA gene. Low methylation status across all CpGs of the nc886 in the young offspring was associated with maternal GDM. While low methylation degree in adult offspring in blood, adipose tissue, and skeletal muscle was not associated with maternal GDM, adipose tissue nc886 expression was increased in O-GDM compared to O-BP, but not in O-T1D. In addition, adipose tissue nc886 expression levels were positively associated with maternal pre-pregnancy BMI (p = 0.006), but not with the offspring’s own adiposity.
Conclusions
Our results highlight that nc886 is a metastable epiallele, whose methylation in young offspring is negatively correlated with maternal obesity and GDM status. The physiological effect of nc886 may be more important in adipose tissue than in skeletal muscle. Further research should aim to investigate how nc886 regulation in adipose tissue by exposure to GDM may contribute to development of metabolic disease.
https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-024-01673-3
Paternal dietary macronutrient balance and energy intake drive metabolic and behavioral differences among offspring
Crean AJ, Senior AM, Freire T, Clark TD, Mackay F, Austin G, Pulpitel TJ, Nobrega MA, Barrès R, Simpson SJ., Nat Commun 2024 Apr; 15(1): 2982.
https://www.nature.com/articles/s41467-024-46782-y/figures/1
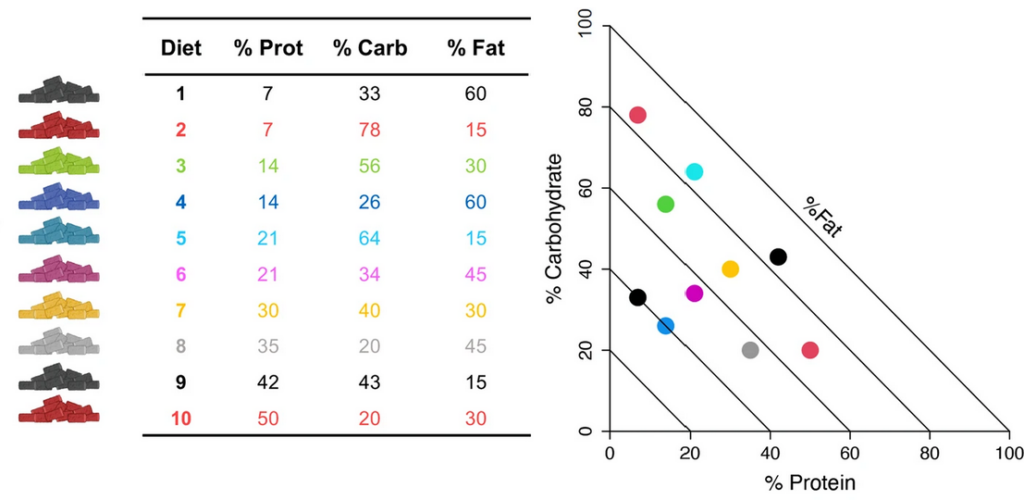
Paternal diet can influence the phenotype of the next generation, yet, the dietary components inducing specific responses in the offspring are not identified. Here, we use the Nutritional Geometry Framework to determine the effects of pre-conception paternal dietary macronutrient balance on offspring metabolic and behavioral traits in mice. Ten isocaloric diets varying in the relative proportion of protein, fats, and carbohydrates are fed to male mice prior to mating. Dams and offspring are fed standard chow and never exposed to treatment diets. Body fat in female offspring is positively associated with the paternal consumption of fat, while in male offspring, an anxiety-like phenotype is associated to paternal diets low in protein and high in carbohydrates. Our study uncovers that the nature and the magnitude of paternal effects are driven by interactions between macronutrient balance and energy intake and are not solely the result of over- or undernutrition.
Dietary macronutrient composition impacts gene regulation in adipose tissue
Farris KM, Senior AM, Sobreira DR, Mitchell RM, Weber ZT, Ingerslev LR, Barrès R, Simpson SJ, Crean AJ, Nobrega MA, Commun Biol 2024 Feb; 7(1): 194.
https://www.nature.com/articles/s42003-024-05876-5
Diet is a key lifestyle component that influences metabolic health through several factors, including total energy intake and macronutrient composition. While the impact of caloric intake on gene expression and physiological phenomena in various tissues is well described, the influence of dietary macronutrient composition on these parameters is less well studied. Here, we use the Nutritional Geometry framework to investigate the role of macronutrient composition on metabolic function and gene regulation in adipose tissue. Using ten isocaloric diets that vary systematically in their proportion of energy from fat, protein, and carbohydrates, we find that gene expression and splicing are highly responsive to macronutrient composition, with distinct sets of genes regulated by different macronutrient interactions. Specifically, the expression of many genes associated with Bardet-Biedl syndrome is responsive to dietary fat content. Splicing and expression changes occur in largely separate gene sets, highlighting distinct mechanisms by which dietary composition influences the transcriptome and emphasizing the importance of considering splicing changes to more fully capture the gene regulation response to environmental changes such as diet. Our study provides insight into the gene regulation plasticity of adipose tissue in response to macronutrient composition, beyond the already well-characterized response to caloric intake.
2023
Splicing across adipocyte differentiation is highly dynamic and impacted by metabolic phenotype in adipose tissue
Nobrega M, Farris K, Andersen E, Donkin I, Versteyhe S, Kristiansen VB, Simpson S, Barres R, Res Sq 2023 Oct
https://www.researchsquare.com/article/rs-3487148/v1
Adipose tissue dysfunction underlies many of the metabolic complications associated with obesity. A better understanding of the gene regulation differences present in metabolically unhealthy adipose tissue can provide insights into the mechanisms underlying adipose tissue dysfunction. Here, we used RNA-seq data collected from a differentiation time course of lean, obese, and obese with type 2 diabetes (T2D) individuals to characterize the role of alterative splicing in adipocyte differentiation and function. We found that splicing was highly dynamic across adipocyte differentiation in all three cohorts, and that the dynamics of splicing were significantly impacted by metabolic phenotype. We also found that there was very little overlap between genes that were differentially spliced in adipocyte differentiation and those that were differentially expressed, positioning alternative splicing as a largely independent gene regulatory mechanism whose impact would be missed when looking at gene expression changes alone. To assess the impact of alternative splicing across adipocyte differentiation on genetic risk for metabolic diseases, we integrated the differential splicing results generated here with genome-wide association study results for body mass index and T2D, and found that variants associated with T2D were enriched in regions that were differentially spliced in early differentiation. These findings provide insight into the role of alternative splicing in adipocyte differentiation and can serve as a resource to guide future variant-to-function studies.
Male reproductive traits are differentially affected by dietary macronutrient balance but unrelated to adiposity
A. Crean, S. Afrin, H. Niranjan, T. Pulpitel, G. Ahmad, A. Senior, T. Freire, F. Mackay, M. Nobrega, R. Barrès, S. Simpson, T. Pini, Nature Communications, 2023, 14 (1), pp.2566. ⟨10.1038/s41467-023-38314-x⟩.
https://www.nature.com/articles/s41467-023-38314-x
Dietary factors influence male reproductive function in both experimental and epidemiological studies. However, there are currently no specific dietary guidelines for male preconception health. Here, we use the Nutritional Geometry framework to examine the effects of dietary macronutrient balance on reproductive traits in C57BL/6 J male mice. Dietary effects are observed in a range of morphological, testicular and spermatozoa traits, although the relative influence of protein, fat, carbohydrate, and their interactions differ depending on the trait being examined. Interestingly, dietary fat has a positive influence on sperm motility and antioxidant capacity, differing to typical high fat diet studies where calorie content is not controlled for. Moreover, body adiposity is not significantly correlated with any of the reproductive traits measured in this study. These results demonstrate the importance of macronutrient balance and calorie intake on reproductive function and support the need to develop specific, targeted, preconception dietary guidelines for males.
2022
Comparative analysis of sperm DNA methylation supports evolutionary acquired epigenetic plasticity for organ speciation
Moharrek F, Ingerslev LR, Altıntaş A, Lundell L, Hansen AN, Small L, Workman CT and Barrès R. Epigenomics. 2022 Nov 24. doi: 10.2217/epi-2022-0168.
Aim: To perform a comparative epigenomic analysis of DNA methylation in spermatozoa from humans, mice, rats and mini-pigs. Materials & methods: Genome-wide DNA methylation analysis was used to compare the methylation profiles of orthologous CpG sites. Transcription profiles of early embryo development were analyzed to provide insight into the association between sperm methylation and gene expression programming. Results: We identified DNA methylation variation near genes related to the central nervous system and signal transduction. Gene expression dynamics at different time points of preimplantation stages were modestly associated with spermatozoal DNA methylation at the nearest promoters. Conclusion: Conserved genomic regions subject to epigenetic variation across different species were associated with specific organ functions, suggesting their potential contribution to organ speciation and long-term adaptation to the environment.
DNA methylation and gene expression in blood and adipose tissue of adult offspring of women with diabetes in pregnancy – A validation study of DNA methylation changes identified in adolescent offspring
Manitta E, Marques ICF, Stokholm S, Kelstrup BL, Houshmand-Oeregaard A, Clausen DT, Grunnet LG, Mathiesen ER, Dalgaard LT, Barrès R, Vaag AA, Damm P, Hjort L. Biomedicines. 2022 May 26;10(6):1244.
Maternal gestational diabetes and obesity are associated with adverse outcomes in offspring, including increased risk of diabetes and cardiovascular diseases. Previously, we identified a lower DNA methylation degree at genomic sites near the genes ESM1, MS4A3, and TSPAN14 in the blood cells of adolescent offspring exposed to gestational diabetes and/or maternal obesity in utero. In the present study, we aimed to investigate if altered methylation and expression of these genes were detectable in blood, as well in the metabolically relevant subcutaneous adipose tissue, in a separate cohort of adult offspring exposed to gestational diabetes and obesity (O-GDM) or type 1 diabetes (O-T1D) in utero, compared with the offspring of women from the background population (O-BP). We did not replicate the findings of lower methylation of ESM1, MS4A3, and TSPAN14 in blood from adults, either in O-GDM or O-T1D. In contrast, in adipose tissue of O-T1D, we found higher MS4A3 DNA methylation, which will require further validation. The adipose tissue ESM1 expression was lower in O-GDM compared to O-BP, which in turn was not associated with maternal pre-pregnancy BMI nor the offspring’s own adiposity. Adipose tissue TSPAN14 expression was slightly lower in O-GDM compared with O-BP, but also positively associated with maternal pre-pregnancy BMI, as well as offspring’s own adiposity and HbA1c levels. In conclusion, the lower DNA methylation in blood from adolescent offspring exposed to GDM could not be confirmed in the present cohort of adult offspring, potentially due to methylation remodeling with increased aging. In offspring adipose tissue, ESM1 expression was associated with maternal GDM, and TSPAN14 expression was associated with both maternal GDM, as well as pre-pregnancy BMI. These altered expression patterns are potentially relevant to the concept of developmental programming of cardiometabolic diseases and require further studies.
https://www.mdpi.com/2227-9059/10/6/1244
Sperm count is increased by diet-induced weight loss and maintained by exercise or GLP-1 analogue treatment: a randomised controlled trial
Andersen E, Juhl CR, Kjøller ET, Lundgren JR, Janus C, Dehestani Y, Saupstad M, Ingerslev LR, Duun OR, Jensen SBK, Holst JJ, Stallknecht BM, Madsbad S, Torekov SS, Barrès R. Human Reproduction. 2022 May 17:deac096.
STUDY QUESTION
Does diet-induced weight loss improve semen parameters, and are these possible improvements maintained with sustained weight loss?
SUMMARY ANSWER
An 8-week low-calorie diet-induced weight loss was associated with improved sperm concentration and sperm count, which were maintained after 1 year in men who maintained weight loss.
WHAT IS KNOWN ALREADY
Obesity is associated with impaired semen quality. Weight loss improves metabolic health in obesity, but there is a lack of knowledge on the acute and long-term effects of weight loss on semen parameters.
STUDY DESIGN, SIZE, DURATION
This is a substudy of men with obesity enrolled in a randomized, controlled, double-blinded trial (the S-LITE trial). The trial was conducted between August 2016 and November 2019. A total of 56 men were included in the study and assigned to an initial 8-week low-calorie diet (800 kcal/day) followed by randomization to 52 weeks of either: placebo and habitual activity (placebo), exercise training and placebo (exercise), the Glucagon Like Peptide 1 (GLP-1) analogue liraglutide and habitual activity (liraglutide) or liraglutide in combination with exercise training (combination).
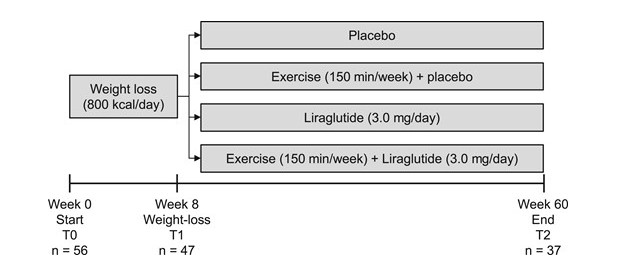
PARTICIPANTS/MATERIALS, SETTING, METHODS
Inclusion criteria were men who delivered semen samples, 18 to 65 years of age, and a body mass index between 32 and 43 kg/m2, but otherwise healthy. The study was carried out at Hvidovre Hospital and at the University of Copenhagen, and the participants were from the Greater Copenhagen Area. We assessed semen parameters and anthropometrics and collected blood samples before (T0), after the 8-week low-calorie dietary intervention (T1), and after 52 weeks (T2).
MAIN RESULTS AND THE ROLE OF CHANCE
The men lost on average 16.5 kg (95% CI: 15.2–17.8) body weight during the low-calorie diet, which increased sperm concentration 1.49-fold (95% CI: 1.18–1.88, P <0.01) and sperm count 1.41-fold (95% CI: 1.07–1.87, P <0.01). These improvements were maintained for 52 weeks in men who maintained the weight loss, but not in men who regained weight. Semen volume, sperm motility and motile sperm count did not change.LIMITATIONS, REASONS FOR CAUTION
The S-LITE trial was a randomized controlled trial of weight loss maintenance. Analysis of semen was preregistered to explore the effects of weight loss and weight loss maintenance on semen parameters, but definite inferences cannot be made.
WIDER IMPLICATIONS OF THE FINDINGS
This study shows that sperm concentration and sperm count were improved after a diet-induced weight loss in men with obesity. Our findings indicate that either or both liraglutide and exercise as weight maintenance strategies may be used to maintain the improvements in sperm concentration and count.STUDY FUNDING/COMPETING INTEREST(S)
This work is supported by an excellence grant from the Novo Nordisk Foundation (NNF16OC0019968), a Challenge Programme Grant from the Novo Nordisk Foundation (NNF18OC0033754) and a grant from Helsefonden. The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent research centre at the University of Copenhagen, partially funded by an unrestricted donation from the Novo Nordisk Foundation (NNF18CC0034900). Saxenda (liraglutide) and placebo pens were provided by Novo Nordisk. Cambridge Weight Plan diet products for the 8-week low-calorie diet were provided by Cambridge Weight Plan. E.A.: shareholder, employee of ExSeed Health Ltd. Grant Recipient from ExSeed Health Ltd and listed on Patents planned, issued or pending with ExSeed Health Ltd; J.J.H.: consultant for Eli Lilly A/S and Novo Nordisk A/S. Lecture fees for Novo Nordisk A/S. Listed on Patents planned, issued or pending with the University of Copenhagen, Advocacy group for Antag Therapeutics and Bainan Biotech; S.M.: lecture fees for Novo Nordisk A/S. Recipient of Support for attending meetings from Novo Nordisk A/S. Advisory boards of Novo Nordisk A/S; Sanofi Aventis and Merck Sharp & Dohme. S.S.T.: research grant recipient Novo Nordisk. The remaining authors have no conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
The trial was approved by the Ethical Committee of the Capital Region of Denmark (H-16027082) and the Danish Medicines Agency (EudraCT Number: 2015-005585-32). ClinicalTrials.gov identifier (NCT number): NCT04122716.
TRIAL REGISTRATION DATE
11 May 2016.
DATE OF FIRST PATIENT’S ENROLMENT
August 2016.
https://academic.oup.com/humrep/article/37/7/1414/6587152?login=true