A human dietary intervention study where participants receive different commonly consumed diet patterns and we are examining the impact it has on epigenetic markers in the sperm. This part of the study is with individual people, singletons.
A human dietary intervention study where participants receive different commonly consumed diet patterns and we are examining the impact it has on epigenetic markers in the sperm. This part of the study is with individual people, singletons.
It is known that the epigenome of sperm cells can be changed when men are exposed to lifestyle changes, such as increased exercise or weight loss. Further, these epigenetic changes have been noted to occur at places on the DNA that may impact the health and development of their children. One lifestyle choice of which we are still working to understand the impact on the quality and epigenome of sperm is diet. Previous research in animals has shown that certain diets in fathers-to-be can potentially lead to altered health outcomes in their children. To help us better understand how the current modern diets of men may be effecting their sperm we designed the Food intake and Epigenetic Alteration in Spermatozoa of Singletons and Twins, also known as the FEASST study.
We will provide male participants with two specific diets, and we will collect health information and biological samples such as blood, semen, and saliva throughout. The diets will consist of a ‘Processed’ and ‘Unprocessed’ version. The aim of these two diets is to study the health effect of consuming a diet matching what men are supposed to eat versus what they are actually eating. The ‘Processed’ diet is based on the average food intake of American men and is made to represent what men of child-rearing age are presently eating and consists of food products that have undergone industrial processing with added sugar, enriched in saturated fat, cholesterol, and sodium. The ‘Unprocessed’ diet is based on the dietary guidelines for men from several countries, including Australia, the US, and Nordic Nations, meant to represent the current nutritional recommendations that are given to young men for optimal health and consists of fruits, vegetables, whole grains, unsaturated fats, lean protein, and fibre.
Participants will be given these diets for three-weeks, followed by a three-month break, and then another three-weeks eating the diet opposite to the one they had during the first three-weeks. Throughout the study various measurements are taken such as: body weight, sperm quality, blood levels of markers related to reproduction and metabolism, and survey information surrounding mental and physical health.
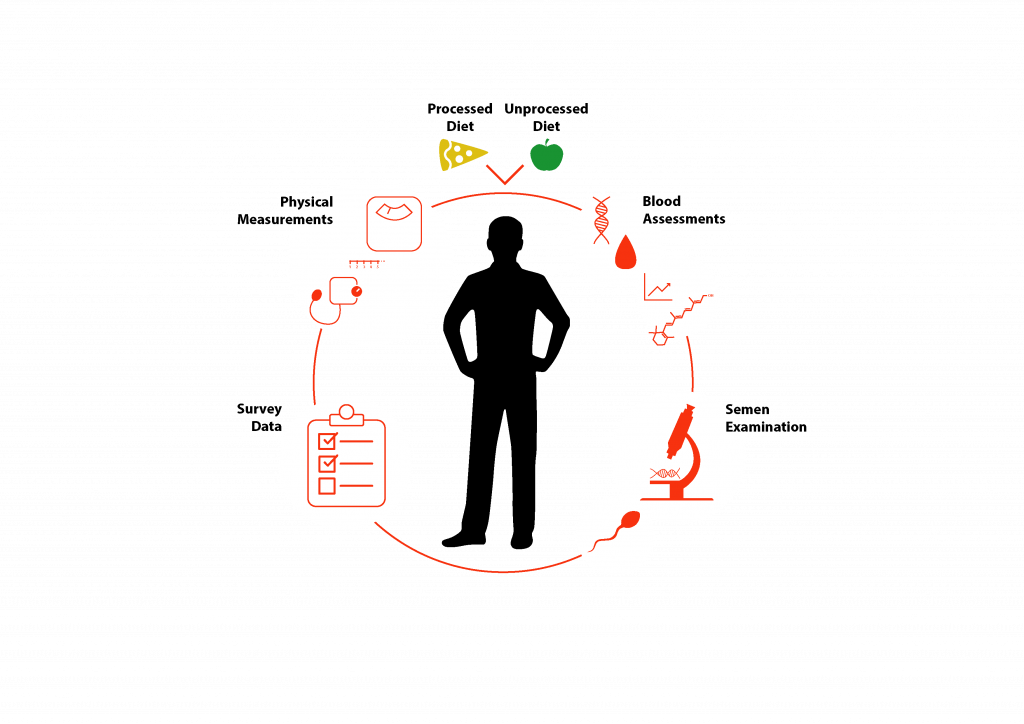
Once the study is completed, blood and semen samples will be further analysed in the lab to identify any epigenetic changes or patterns. We will look at epigenetic changes such as presence of RNA species and epigenetic-modifying features that interact with gene expression. We will analyse data from these experimental procedures to determine the relationship of different diets on the characteristics of sperm
Jessica Preston was hired by the GECKO consortium with funding from The Copenhagen Bioscience PhD Program to conceptualize and plan the FEASST Singletons and Twins studies.
Jessica was officially enrolled as a PhD Student at the Novo Nordisk Foundation Centre for Basic Metabolic research, University of Copenhagen
The FEASST Singletons study and all accompanying research study documents were submitted to the Capital Regions of Denmark Committee on Health Research Ethics
Comments on the original study documents were received, suggesting changes and amendments to the study design and documentation in order to ensure compliance with health research ethical standards.
Study documents were edited in accordance with the ethics committee’s suggestions and re-submitted as an amendment for further ethical review*.
*Processing of reviewing a research study to determine fulfilment of a country or institution’s criteria for the conduct of ethical research.
The research study received official approval from the Region Hovedstaden Videnskabsetiske komitéer (Copenhagen Capital Region Research Ethics Committee) in order to begin study conduct.
Information on ethical approval can be found here: https://clinicaltrials.gov/study/NCT05368194
Recruitment of study participants commenced, including publicising the study, screening applicants, and determining the fulfilment of eligibility criteria* by potential participants.
*The specifications that an individual must meet in order to be included in a clinical research study, such as a specified sex, age range, or health condition.
The selected study participants began the nutrition intervention* and attended a series of 6 appointments at the study center to assess health changes.
*A type of research study in which research participants are given a specific diet for a set period of time.
The nutrition intervention was complete, and all study participants finished their dietary period and attended all study appointments
Statistical analysis conducted on all health information collected throughout the nutrition intervention.
Laboratory-based measurements on frozen biological materials* from study participants was completed to determine the levels of various haematological parameters** and epigenetic parameters***.
* Human tissues collected from study participants, including blood, saliva and semen.
** The levels of various compounds within the blood used to determine physiological and health status.
***The pattern and frequency of changes to the DNA regulation, including the levels of DNA methylation and smallRNA expression.
All laboratory-based measurement of the collected tissues completed.
Biostatistical* interpretation of the complex parameters assessed from the study participant biological material, including statistical analysis of epigenetic parameters and metabolomic parameters**.
*Statistical approaches related to biological data sets such as a clinical research study
**The measurement of the variety of compounds involved in metabolism identified in the biological materials.
Statistical interpretation of all data sets pertaining to the study is anticipated being completed.
Anticipated publication of the study results and submission of the study leader’s PhD.

TEs (Transposable Elements), escape epigenome reprogramming and therefore represent potential hotspots of heritable information that can be passed on to future generations. The Latin suffix –theca (from Ancient Greek thēkē) is used for any kind of collection. The goal of the GeckoTEk project is to generate a near-complete human sperm (epi)genome of high-quality, using third-generation sequencing technologies with a particular focus on difficult genomic regions and repetitive sequences.
An ex vivo characterisation of tissue specific epigenetic remodelling in offspring sired from nutritionally challenged fathers. We utilise approaches such as HI-C and ATAC-seq to develop a picture of genetic architecture, and integrate chromatin confirmation and transcriptomic data to determine how epigenetic regulatory elements reshape the genome.
EpiPIG is a research study in which we are giving mini-pigs either a ‘Western’ high fat/high sugar diet or a normal pig diet to better understand what epigenetic impact this has on their sperm.
A human dietary intervention study where participants receive different commonly consumed diet patterns and we are examining the impact it has on epigenetic markers in the sperm. This branch of the study has twin male participants.
A dietary intervention study in which male mice are given one of 10 diets with different proportions of protein, fat and carbohydrates, and then mated to produce offspring. Following which, we examine the effect of these different diets on the overall health and behaviour of both the males and their offspring. We are especially looking for an epigenetic patterns.
A dietary intervention study building on the results from the first geoMOUSE project. Here, one of three isocaloric diets of varying macronutrient compositions are given to male mice, which are then mated to produce offspring. This study aims to extend the investigation on whether offspring epigenetic profiles are influenced by paternal nutrition and how this affects behavioral and cognitive outcomes.
A dietary intervention study with guinea pigs fed high or low –fat diets with or without additional Vitamin C to identify through which mechanisms nutritional factors influence epigenetic inheritance of obesity and metabolic disease.
A partnership with Taronga Zoo and the Copenhagen Zoo to assess sperm epigenetic signatures across a wide range of species. The study aims to build a reference map of sperm epigenome modifications among animal species to understand similarities and differences in what environmental information is transmitted in sperm.
SEAS = Sperm Epigenomics Across Species
Endurance training remodels sperm-borne small RNA expression and methylation at neurological gene hotspots.
We exposed young men to a 6-week endurance training exercise regime, and measured the epigenetic signature of their sperm before and after the intervention. This study highlighted exercise-induced remodelling of genes involved in the brain.
The epigenetic signature of both lean and obese men, and men before after weight loss was examined, highlighting both rapid and long-term remodelling of the sperm epigenome at gene regions involved in appetite regulation.